After being turned down by four food microbiologists to write a guest column on the effect of the microbiome on obesity, I was referred to a member of the faculty of the Department of Foods and Nutrition at the University of Georgia who is conducting research in this area. All of my interactions with Dr. Claire de La Serre have been by electronic communication. Although this post is more technical than most published to date on this site, I hope that you will find it much more illuminating than what we have learned in popular media.
by Claire de La Serre
The term microbiota (sometimes refers to as microflora) encompasses all the microorganisms that share our body space: bacteria, viruses but also archaea and fungi. They are located in our guts, on our skin, in our mouths, vaginas etc. The vast majority of these microorganisms are bacteria and most of them live in our gastrointestinal (GI) tract.
There has been a lot of research and media coverage on the role the gut microbiota may be playing in maintaining or impairing health, and according to some, gut bacteria are either the cause or the cure to all diseases. Gut bacteria feed on the food we consume and perform critical functions for their host (us). For example, they are our main producer of vitamin K, which is critical for blood coagulation. Gut bacteria also digest otherwise indigestible carbohydrates and the subsequent fermentation products are used by our gut cells for energy, growth, etc. They are also critical in establishing and maintaining immune function. So, it is not surprising that a non-optimal or deleterious microbiota composition could have an impact on the host.
The nature of the bacteria present in our gut and their relative abundance is directly influenced by our environment: the germs we are exposed to, the house we live in, our antibiotic usage etc. The main modulator of gut bacteria composition is the food we eat. The quality and quantity of food consumed has a direct influence on which type of—and how much—bacteria grow in the GI tract. There is evidence that the current typical western diet may not be optimal for gut bacteria composition. Alterations in microbiota composition in response to these diets may be contributing to excessive body weight gain.
A potential role for the microbiota in obesity was found in animal studies. Some laboratory animals are raised in sterile conditions and lack a microbiome. These germ-free rodents can then by colonized with a specific microbiota. Interestingly, germ free mice that receive a western type diet microbiota gain significantly more weight than germ-free mice that received a “normal” type microbiota. A gut microbiota composition that is potentially harmful to its host is referred to a dysbiosis. Animal studies have found that diet-driven dysbiosis can increase the amount of energy extracted from food, enhance the absorption of energy, facilitate fat deposition and prevent satiety. All of these outcomes potentially result in weight gain.
Hunger and satiety are regulated by different brain feeding centers that may be influenced by gut bacterial. Data show that bacteria can produce neurotransmitters such as serotonin and dopamine that may directly affect neuronal function. There is actually a direct neural connection from the gut to the brain: the vagus nerve. Additionally, bacteria may affect brain function indirectly by promoting low grade inflammation, especially at the level of the vagus nerve.
Vagal inflammation is accompanied with remodeling of the brain feeding centers in animal models of dysbiosis. Interestingly, a lot of chronic diseases that have been linked to microbiota composition have an inflammatory component, whether it is metabolic diseases such as obesity and diabetes, neuronal dysfunctions, or autoimmune disorders. Diet-driven dysbiosis is associated with an increase in the inflammatory potential of the microbiota, including the production of bacterial compounds. In addition, gut integrity is altered and the GI tract becomes leaky allowing these pro-inflammatory compounds to enter the circulatory system where they can potentially affect different organs, including the brain.
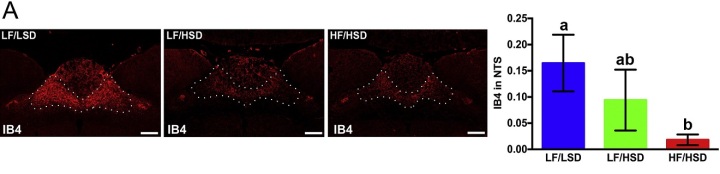
Consumption of diets rich in fats and sugars induces withdrawal of sensory fibers within the hindbrain. These fibers are responsible for transmitting post-ingestive information to the brain and regulating meal size. A. Representative coronal sections of the intermediate NTS (Feeding center, outlined) revealing more IB4-immunoreactive fibers in rats fed with LF/LSD (low fat/low sugar diet) compared to LF/HSD or HF/HSD for four weeks. Quantification on the left shows binary analysis of the area of IB4-labeled vagal afferents in the NTS by using Nikon Element’s binary imaging analysis software; n=4 per group. The one-way ANOVA with a Tukey-Kramer post-hoc correction shows a significant decrease in IB4-labeled vagal afferents in HF/HSD compared to that of LF/LSD (p≤0.05). All data are expresses as average±SEM; Mean values at week 4 with unlike letters were significantly different. Scale bar =250µm. Adapted from Sen et al, Physiology and Behavior, 2017. Reproduced with permission.
The diets used in these studies are refined diets with a set macronutrients make-up. They are generally high in saturated fat; 45% to 60% of kcal coming from fat is common. 45% of energy coming from fat is not necessarily unheard of for humans but for a laboratory rodent, it is pretty high. Interestingly, diets rich in sugars (sucrose or fructose) appear to have very similar effects on rodents. It is important to note that these obesogenic diets are also very low in fiber, particularly soluble fiber.
Soluble fiber is the main source of fermentable carbohydrates for our gut bacteria. Recent studies have found that when bacterial fermentation does happen by supplementing diets with soluble fiber or mimicking its effect via delivery of fermentation products, it has a protective effect against obesity and pre-diabetes. This may be due to the protective role of fermentation products on the intestinal barrier. If the GI tract is less leaky, less bacterial inflammatory products are getting into the bloodstream.
In addition to the data discussed above, there are a lot of studies showing changes in microbiota composition with different pathologies, in both humans and animals. It is critical to keep in mind that correlation does not necessarily imply causation; it is not because the microbiota composition changes in certain disease states that bacteria are responsible for that disease. Changes in microbiota composition can be an accompanying symptom, it could also have no direct effect on host health. The microbiota is composed of thousands and thousands of bacteria. New technologies such as next generation sequencing have allowed for a more complete understanding of the bacterial makeup.
Any changes in environmental conditions, which are likely to happen in disease states, are expected to produce modifications in microbiota composition. Causality can be hard to determine in microbiota studies and is often limited to germ free studies where animals can be inoculated with a specific microbiome independently of the environmental pressures that have resulted in the bacterial make up. It is important to keep in mind that germ free animals differ in their metabolism, immune system and neurological functions from conventionally bred animals because they have never been exposed to germs before. Therefore, attempting to deplete the resident microbiota via antibiotics followed by microbial colonization may be a better approach. Such studies have been done and confirm the idea that the microbiota affect host metabolism and that western diet-driven dysbiosis participates in weight gain.
What does this mean for human health? Are we all doomed to suffer from microbiota-driven chronic metabolic disease? Some animal models of dysbiosis do not gain excessive weight and maintaining a healthy gut and strong intestinal barrier may be key. Prebiotics, probiotics, antibiotics and supplementation with fermentation products have all add beneficial effects on host physiology, especially glycemic control. A common pathway may be the protective role of fermentation products on the intestinal barrier and a reduction in inflammation.
Most of these studies were again done in animals, and some human data is promising but the effect appears to be short lived. This is one of the important questions in microbiota research, how translational is it? Can we actually manipulate our microbiome to our own advantage? There have been attempts at microbiota replacement in humans, notably via fecal transfer and they have been quite successful in treating or at least alleviate symptoms of chronic gut inflammatory conditions. The general make-up of the gut bacteria a reflection the best approach for improving microbiota composition is likely to increase fiber consumption.
Dr. de La Serre is an Assistant Professor of Foods and Nutrition at the University of Georgia. Her doctorate in Physiology and Nutrition is from AgroParisTech, and she completed a Postdoctoral Research Fellowship at Johns Hopkins. She directs the Gastrointestinal Neurophysiology Laboratory which studies the pathways by which diet composition affects energy balance. She is particularly interested in the influence of energy-dense diets on gut microbiota composition, gastrointestinal (GI) functions and inflammation. She studies how changes in microbiota composition can affect gut-brain signaling to promote overeating. Her laboratory uses animal models and studies phenomena from the behavioral aspect to the molecular pathway. In addition, she teaches a course in Metabolism and Physiology of Food Intake, Energy Balance and Obesity, a course I would love to take!
BTW, Dr. Yoni Freedhoff lists “microbiome” as one of his 83 weasly, quactackular words that we should not be using in print. I note that Dr. de La Serre refers to microbiota in her article, and I will try to refrain from using microbiome in the future.
Next week: Why the BMI is not useful for postmenopausal women and the elderly
Reference:
Sen T, Cawthon C, Ihde BT, Gawey BJ, Ahmed M, Long JA, Kirkland R, Hajnald A, DiLorenzo PM, de La Serre CB, Czaja K. Diet-driven microbiota dysbiosis is associated with vagal remodeling and obesity, Physiology and Behavior 173 (2017) 305-317.

Great website. Plenty of helpful info here. I am sending it to some friends ans also sharing in delicious. And obviously, thanks for your effort!
LikeLike